We put excellence, value and quality above all - and it shows




A Technology Partnership That Goes Beyond Code

“Arbisoft has been my most trusted technology partner for now over 15 years. Arbisoft has very unique methods of recruiting and training, and the results demonstrate that. They have great teams, great positive attitudes and great communication.”
A Blueprint for Smarter Innovation: The 4 Pillars of Modern, AI-Fueled Healthcare Innovation

Healthcare innovation has historically been a slow and arduous process. Bringing a new medical device to market, for instance, has traditionally taken three to seven years. According to a 2023 McKinsey analysis, the journey from concept to market for a new medical device has historically averaged three to seven years. This lengthy timeline, filled with endless meetings, iterative testing cycles, and complex regulatory hurdles, has had a real human cost.
The true cost of this inertia was measured not in time, but in foregone patient outcomes. This delay directly translates to prolonged reliance on inferior diagnostics for patients and outdated treatment paradigms for physicians. With each passing year, the disconnect between the art of the possible in medicine and the reality of clinical practice became more pronounced.
The pace of change in healthcare innovation is accelerating. Innovation cycles that once spanned years are now being completed in just months. This shift reflects not only advances in technology but also a fundamental change in how healthcare organizations approach innovation. According to Forrester’s report, The State of Generative AI in Healthcare, 2024, 46% of U.S. healthcare organizations are currently in the early stages of implementing Generative AI. This signals a move beyond isolated experiments toward a fundamental rewiring of the innovation process itself.
The integration of Artificial Intelligence is delivering a tangible competitive advantage in healthcare innovation, marked by streamlined research, superior accuracy, and radically shortened timelines. This is not a future promise but a present reality, as demonstrated by front-runners like Google Health (e.g., with AI for diagnostics) and device makers like Boston Scientific, which are leveraging digital twins and advanced simulations, slashing the time and cost of physical prototyping.
To understand how to harness this shift, this article will guide you through the four stages of healthcare innovation, detailing the transformative impact of AI on each to achieve faster and more intelligent outcomes.
Understanding Innovation in Healthcare
Innovation in healthcare is often misunderstood. Many people assume that innovation only means invention—the creation of something entirely new or highly advanced. While breakthrough technologies and novel medical devices are certainly forms of innovation, they represent only a small part of what true healthcare innovation encompasses.
In reality, innovation is any change that adds value to healthcare, whether it is clinical, operational, technological, or patient-facing. An innovation does not have to be complex or expensive; it simply needs to make healthcare delivery better, safer, or more efficient. This can include making processes more automated, services more accessible, care more affordable, experiences more convenient, or workflows more streamlined.
Innovation can be something enhanced, improved, or simpler—not necessarily something brand new. It may make healthcare faster, safer, cheaper, or more valuable for patients, staff, and organizations alike.
By broadening the definition of innovation beyond invention, healthcare organizations can unlock thousands of practical opportunities to solve problems, reduce costs, improve outcomes, and elevate patient and clinician experience.
The Four Phases of Healthcare Innovation
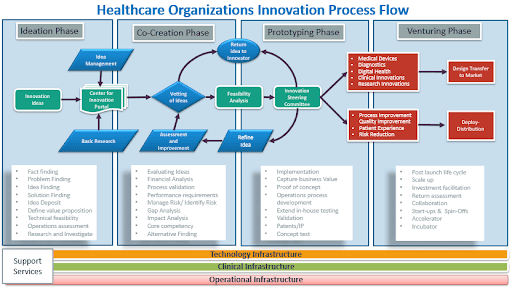
Every healthcare innovation moves through four phases:
- Ideation: Teams come up with ideas and conduct basic research to identify problems worth solving.
- Co-Creation: Doctors, engineers, and patients work together to develop and refine the concept.
- Prototyping: Teams build test versions to see what works and what needs improvement.
- Venturing: The solution launches to hospitals and patients.
The underlying factor in achieving successful innovation in healthcare is not only the innovation process itself but the strength of the environment that supports it. Ultimately, all four phases of innovation rely on three critical foundational infrastructures:
The underlying factor in achieving successful innovation in healthcare is not only the innovation process itself but the strength of the environment that supports it. Ultimately, all four phases of innovation rely on three critical foundational infrastructures:
- Technology Infrastructure: Modern, integrated digital systems, data platforms, and interoperability capabilities that allow AI, automation, and analytics to function reliably and securely.
- Clinical Infrastructure: Standardized clinical workflows, evidence-based practices, and clinician engagement models that ensure innovations are safe, adoptable, and aligned with patient care needs.
- Operational Infrastructure: Streamlined administrative, financial, and organizational processes that enable scalable implementation, governance, and sustained performance improvement.
Together, these foundations create the conditions where innovation can move beyond ideas and pilots, enabling healthcare organizations to deliver faster, safer, more affordable, and more impactful outcomes.
Technology infrastructure provides the computer systems and software needed for innovation. Clinical infrastructure provides the hospitals, research facilities, and medical expertise. Without both, innovation stalls.
For decades, this process moved slowly. Teams spent months debating without clear answers. They built expensive prototypes that often failed. They launched products hoping they would work, often guessing wrong. The whole process felt exhausting and uncertain.
Now, AI and data analytics are changing how each step works. The steps remain the same, but everything moves more smoothly. Let’s look at how this happens, starting with where every innovation begins: finding the right problem to solve.
Phase One: Ideation
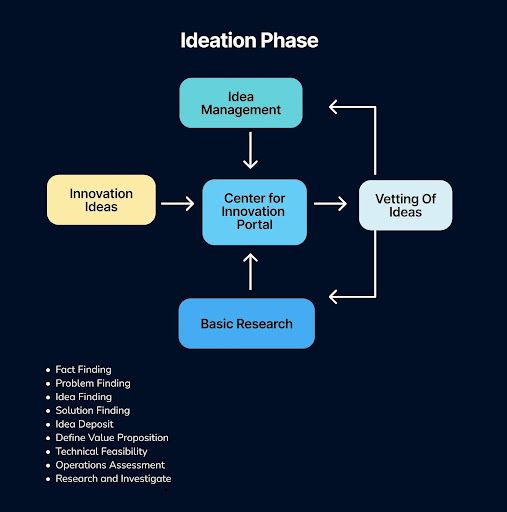
The initial phase of innovation is a critical, foundational period that extends far beyond the simple generation of ideas. It is a disciplined stage of fact-finding and intense research and investigation, dedicated to deeply understanding the landscape and pinpointing the core challenge through rigorous problem-finding. This stage is about exploration and definition: teams engage in idea finding and solution finding to generate potential pathways, often capturing these early concepts in a central idea deposit.
Crucially, this phase also involves the initial vetting of these possibilities, beginning to define the value proposition, and conducting preliminary assessments of technical feasibility and operations to separate promising concepts from mere possibilities. It is this comprehensive groundwork that transforms vague inspiration into a structured pipeline of viable opportunities, setting the stage for all subsequent development and execution.
In the past, healthcare innovation started with gut feelings. A doctor noticed something wrong and proposed a solution based on personal experience. This worked sometimes, but it was limited. One person can only see so much.
Today, AI looks at millions of patient records at once. It reads through clinical notes, research papers, and treatment outcomes. It finds patterns that no human could spot alone. AI can identify which problems affect the most people, which treatments have the biggest gaps, and where the healthcare system is failing patients.
This changes everything about how innovation starts. Teams no longer spend six months researching whether a problem is worth solving. AI does that work in weeks. Instead of pursuing interesting ideas, teams focus on problems that data proves truly matter to patients.
The research phase becomes much shorter. Finding facts, validating problems, and understanding what solutions might work used to take half a year. Now it takes a few weeks because AI handles the heavy analysis work. Once teams know exactly what problem they need to solve, they face the next challenge: getting everyone to agree on how to solve it.
What to Measure
Objective | What to Measure | Why It Matters |
| Validation efficiency | Average time to move from hypothesis to data-verified need | Tracks how efficiently teams validate ideas using data evidence |
| Problem relevance | % of innovation proposals backed by measurable clinical or operational data | Ensures focus on validated, high-impact problems |
| Insight coverage | Number of distinct data domains used for validation (e.g., EHR, patient feedback, workflow data) | Ensures problem selection is informed by diverse, real-world evidence |
| Strategic alignment | % of validated problems mapped to strategic or population health priorities | Confirms innovation supports business and health system objectives |
| Impact potential | Weighted impact score (clinical value × patient reach × cost efficiency) | Prioritizes problems that deliver measurable patient and business value |
Phase Two: Concept Development & Screening
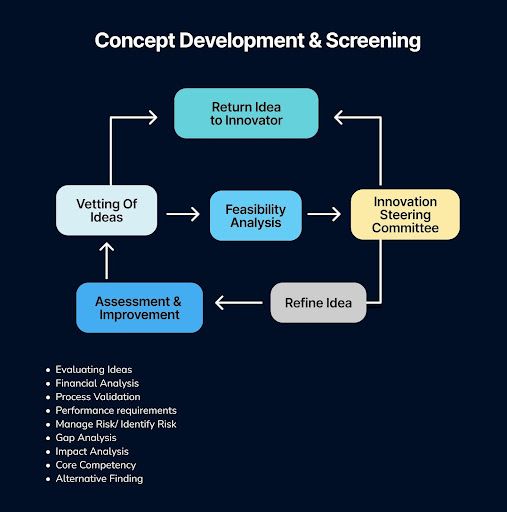
The second phase of innovation, Concept Development & Screening, marks the critical transition from raw ideas to actionable concepts. This stage is far more than a simple vetting of ideas; it is a rigorous, multi-dimensional assessment designed to build viability and alignment.
Promising ideas are systematically developed and stress-tested through a comprehensive evaluation of performance requirements, financial analysis, and potential impact. It involves a crucial gap analysis to identify missing capabilities, a review of core competency alignment, and proactive efforts to manage and identify risk.
Furthermore, this phase includes process validation to gauge feasibility and encourages alternative findings to ensure the most robust solution is advanced. Ultimately, this disciplined process transforms abstract possibilities into a curated portfolio of well-defined concepts, ready for the significant investment of the subsequent development stage.
Once there’s a clear problem, different people need to work together to solve it. This is where things traditionally got messy. Doctors wanted the best patient outcomes. Engineers cared about what was technically possible. Hospital administrators worried about costs. Patients wanted something simple and accessible.
Getting all these people to agree used to mean months of meetings. Everyone had opinions. Everyone thought they were right. Progress crawled forward through endless debates and compromises.
AI and data change this completely. When AI maps out how patients actually experience healthcare, it shows everyone the same objective picture. Instead of arguing about opinions, teams look at evidence together. They see exactly where the bottlenecks are, what’s causing delays, and what patients actually need.
AI can also create quick mock-ups of potential solutions. This gives teams something concrete to discuss from the very beginning. Financial analysis happens faster. Risk assessment becomes clearer. Everyone works from the same information.
This evidence-based approach cuts through disagreements. For instance, when a heart surgeon and a software engineer both see data showing that diagnoses get delayed by 40%, they stop arguing and start solving that specific problem together.
Similar data-driven collaboration is reshaping diagnostics, too. Deep learning for medical imaging is helping doctors and engineers co-create faster, more reliable diagnostic tools, guided by real patient data rather than assumptions.
What used to take months of vetting ideas and debating feasibility now takes weeks. Teams assess financial viability, evaluate impact, and identify risks using data tools. They can compare different solution options side by side using predictions instead of guesses. With everyone aligned on what to build, the team moves into what has traditionally been the most expensive phase: building and testing the solution.
What to Measure
Objective | What to Measure | Why It Matters |
| Alignment speed | Average time from idea proposal to consensus on scope | Indicates how fast teams reach agreement to move forward |
| Decision quality | % of key decisions supported by shared analytics or validated evidence | Measures strength of evidence-based decision-making |
| Cross-functional engagement | Number of stakeholder groups actively using shared dashboards or simulations | Shows how effectively data tools are bridging silos |
| Decision stability | % of major decisions unchanged after first review | Reflects clarity, confidence, and alignment in early decisions |
| Data accessibility | % of stakeholders with real-time access to shared data | Indicates maturity of data democratization and collaboration quality |
Phase Three: Implementation (Prototyping & Development)
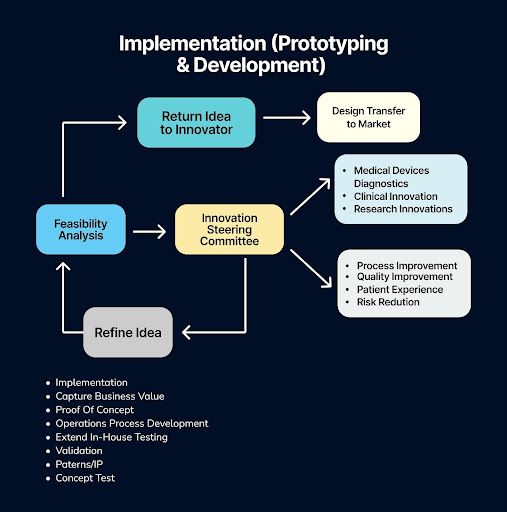
The Implementation (Prototyping & Development) Phase marks the critical transition from abstract ideas to tangible value creation. This stage is dedicated to building, testing, and refining a working model—moving beyond the "what if" to answer "does it work?" Through activities like prototyping, proof-of-concept development, and extended in-house testing, the goal is to achieve validation of the core concept and its practical feasibility.
It is here that theoretical potential meets operational reality, as operations process development begins, patents and IP are secured, and a final concept test confirms the solution's viability. Ultimately, this entire phase is strategically focused on one objective: to capture business value by de-risking the innovation and building a solid foundation for scaling and launch.
Building prototypes has always been the most expensive and time-consuming part of innovation. A single prototype of a medical device can cost hundreds of thousands of dollars. Each new version takes months to build, and most prototypes fail. You only find out what’s wrong after spending all that money and time.
This is where AI creates the biggest transformation. Instead of building physical prototypes right away, teams test everything digitally first.
For software and apps, AI can create user interfaces and simulate how thousands of people would use them. For physical devices and new drugs, AI builds what are called digital twins. A digital twin is a computer simulation that behaves exactly like the real thing would. You can test it under any condition you want without building anything physical.
For example, imagine designing a new pacemaker. Instead of building one and testing it, you create a digital version first. You run it through thousands of simulations, test it under different conditions, and simulate years of wear and tear. You find every potential failure point. All of this happens on a computer in days instead of months.
This approach works for everything. Medical devices, diagnostic tools, phone apps, clinical procedures, and even process improvements can all be tested virtually. Teams can simulate how a new hospital check-in process would work or how a new patient monitoring system would perform without disrupting real operations. Similar ideas are already shaping real healthcare workflows; for instance, AI agents are being built to handle patient scheduling, triage, and follow-up care, turning everyday processes into intelligent, connected systems.
When teams build virtual prototypes, they fail fast and learn fast. A flaw that would cost six months and half a million dollars to find in a physical prototype gets caught in a week-long simulation. By the time teams build something physical, they already know it will work. They build the winning version first instead of the third or fourth try.
Testing, validation, and partnership discussions all move faster because people can see working simulations instead of sketches on paper. By the time something gets built physically, most of the risk is already gone. But even the best-tested product faces one final hurdle: convincing the market to adopt it.
What to Measure
Objective | What to Measure | Why It Matters |
| Simulation-to-build ratio | Number of virtual prototypes tested before any physical build | Shows efficiency of using AI simulation to reduce costs and speed testing |
| Iteration cycle time | Average time from insight to test validation | Measures innovation agility and responsiveness |
| Cost per validated learning | Total prototype spend divided by number of validated insights | Links experimentation to tangible learning outcomes |
| Predictive accuracy | % correlation between simulation results and real-world test outcomes | Evaluates reliability of AI-driven digital twin simulations |
| Compliance readiness | % of prototypes meeting regulatory documentation standards at first submission | Reduces rework and accelerates regulatory approval cycles |
Phase Four: Commercialization & Diffusion Phase
Navigating the complexities of the Commercialization and Diffusion Phase in healthcare—from scaling proven AI/ML solutions and facilitating investment to managing the post-launch lifecycle—requires a rigorous, systematic approach to overcome industry-specific hurdles like regulatory oversight and integration with clinical workflows.
Success in this critical stage is not merely a function of a great innovation but depends on a strategic partnership with a technology provider capable of facilitating robust scale-up, accurate return assessment, and seamless collaboration. This is where a partner with deep expertise in engineering reliable, compliant, and scalable AI systems becomes indispensable, transforming theoretical potential into measurable real-world impact and sustainable commercial success. To explore how our proven methodology can de-risk your innovation's journey to market, visit arbisoft.com.
Many products failed after launch despite careful planning. Organizations invested everything and then hoped for the best.
AI changes launch from guessing to knowing. Predictive analytics look at how similar innovations performed in the past. They forecast adoption based on how ready the market is, whether insurance will cover it, and what competing products exist. Teams can simulate different launch strategies and see which approach works best before committing.
This matters enormously for everyone involved. Investors see less risk and feel more confident providing funding. Hospital systems feel better about adopting new solutions. Return on investment calculations rest on solid projections instead of hopes.
Planning for scale-up, working with early adopter hospitals, and transferring from development to market all become more strategic. Potential problems get identified before launch instead of after. Deployment plans account for real constraints that AI analysis identified.
Once the solution reaches the market, the work doesn’t stop. Post-launch lifecycle management, scale-up, and collaboration with accelerators or incubators ensure continued improvement, support, and investment facilitation for sustained success.
The result is straightforward: solutions reach patients months or years earlier, and they’re much more likely to be used and actually help people.
What to Measure
Objective | What to Measure | Why It Matters |
| Adoption predictability | % variance between forecasted and actual adoption rates | Tracks accuracy of AI-based adoption forecasts |
| Market readiness accuracy | Precision of readiness indicators (regulatory, operational, and demand-related) | Prevents premature or delayed product launches |
| Return velocity | Time from market entry to measurable clinical or financial impact | Connects innovation directly to patient outcomes and ROI |
| Scale efficiency | Cost and time to expand from pilot to full deployment | Measures scalability and resource optimization |
| Feedback loop speed | Time from market launch to first iteration or update based on real-world data | Reflects agility in post-market learning and improvement |
This entire transformation, from finding problems to launching solutions, raises an important question: where do humans fit in all of this?
How a Partner Leverages AI/ML for Commercialization
A proficient technology partner can deploy AI/ML to drive success across your commercialization goals. The table below links specific AI capabilities to commercialization outcomes, with examples from the field:
Commercialization Goal | AI/ML Capability | Real-World Impact & Examples |
| Scale-Up & Post-Launch Lifecycle | Predictive Analytics & Remote Monitoring | AI analyzes patient data to predict health risks and monitor chronic conditions remotely. Biofourmis uses wearables and AI for continuous monitoring, helping to reduce hospital readmissions. |
| Investment Facilitation & Return Assessment | Operational & Administrative Automation | AI automates tasks like clinical documentation and billing. Abridge's ambient documentation solution, deployed at Kaiser Permanente, reduces documentation time by over 50%. Nuance Dragon Medical One automates clinical transcription. |
| Collaboration & Integration | Seamless Health Data Integration | AI agents are built to integrate with EHRs and imaging systems, pulling and analyzing data without disrupting clinical workflows. |
| Start-ups & Spin-Offs | Accelerated Drug Discovery & Development | AI analyzes vast datasets to identify drug candidates and predict efficacy. Insilico Medicine uses AI for drug discovery and to accelerate clinical trials. |
Barriers to Innovation in Healthcare Organizations
Successful healthcare innovation is often hampered by a confluence of strategic, cultural, and structural barriers. Fundamentally, many organizations operate with a lack of a coherent strategy and vision for innovation, which is exacerbated by leadership's expectation of unrealistic, quick payoffs and a short-term focus driven by shrinking margins. This is compounded by the absence of a systematic innovation process, leading to fragmented efforts that are often structurally and functionally separated from day-to-day operations.
Internally, a high-stress environment with low employee engagement fosters cultural resistance, internal politics, and a general lack of interest. Furthermore, a misalignment between leadership and the workforce, combined with a lack of structured incentives, fails to foster a culture of experimentation. Externally, organizations must navigate stringent regulatory oversight and intellectual property complexity, while also struggling to integrate rapid technological advancements that outpace evolving clinical workflows and staff skills. Ultimately, without a shared commitment and a structured approach, innovation efforts remain superficial and unsustainable.
The New Reality of Healthcare Innovation
Healthcare innovation has fundamentally accelerated. The same four phases still exist, but the timeline has collapsed. Problems get identified in weeks instead of months. Teams align in weeks instead of months. Prototypes get validated in days instead of quarters. Launches happen with confidence instead of anxiety.
For patients, this acceleration means receiving life-changing solutions years earlier. A treatment that would have taken four years to validate takes eight months. Every month saved multiplies the human impact.
This isn’t about rushing through the old-school process. It’s about building a new process where data shows the path forward, AI removes the obstacles, and human expertise guides the direction. The result is innovation that’s both faster and smarter, always keeping patients at the center of every decision.
Accelerating Healthcare Innovation with Arbisoft
The acceleration of healthcare innovation isn’t just theoretical; it’s already happening. Globally, the most referenced advancements in AI for healthcare—including AI-driven diagnostics, predictive analytics, precision medicine, drug discovery, remote monitoring, operational automation, and strong governance around ethics, data privacy, and explainability—are reshaping how care is delivered and how organizations operate. These trends demonstrate that innovation is no longer limited to invention alone; it now includes anything that makes healthcare faster, safer, more accessible, more affordable, more connected, and more valuable.
At Arbisoft, we’re helping make this transformation real. By combining advanced AI engineering with deep healthcare domain expertise, Arbisoft partners with medical organizations, research teams, and healthtech innovators to turn these high-impact use cases into working solutions. From developing intelligent diagnostic systems and predictive models to building secure, scalable data platforms and AI governance frameworks, Arbisoft’s work exemplifies how human expertise and machine intelligence come together to create breakthroughs that reach clinicians and patients sooner.
Whether your organization is exploring diagnostic AI, predictive tools, workflow automation, or enterprise-scale data modernization, Arbisoft helps you move from concept to clinical impact faster than ever before.
If your organization is exploring how to accelerate innovation with AI, contact us today to start building smarter, faster, and more impactful healthcare solutions with Arbisoft.
























